NEET-XII-Chemistry
c2022 year:2022
- Qstn #30The given graph is a representation of kinetics of a reaction.
 The `` y `` and `` x `` axes for zero and first order reactions, respectively are
The `` y `` and `` x `` axes for zero and first order reactions, respectively are
(A)zero order ( `` y= `` rate and `` x= `` concentration), first order `` \left(y=\right. `` rate and `` \left.x= t _{1 / 2}\right) ``
(B)zero order ( `` y= `` concentration and `` x= `` time), first order ( `` y= t _{1 / 2} `` and `` x= `` concentration)
(C)zero order ( `` y= `` concentration and `` x= `` time), first order `` (y= `` rate constant and `` x= `` concentration)
(D)zero order ( `` y= `` rate and `` x= `` concentration), first order `` \left(y= t _{1 / 2}\right. `` and `` x= `` concenration `` ) ``digAnsr: CAns :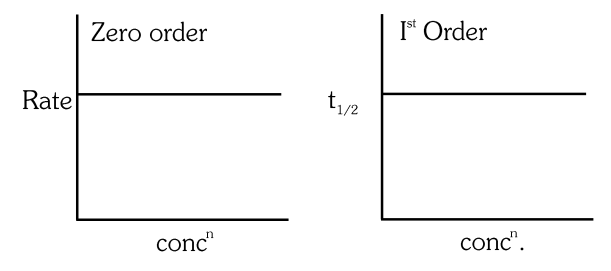
(I) curve is suitable for zero order if `` y= `` rate and `` x = `` concentration because in case of zero order reaction rate is constant and does not depend on conc `` ^{ n } `` .
(II) curve is suitable for first order if `` y=t_{1 / 2} `` and `` x= `` conc `` ^{n} `` because in case of first order `` t_{1 / 2} `` does not depend on conc `` ^{ n } `` .
- Qstn #31The incorrect statement regarding chirality is :
(A)A racemic mixture shows zero optical rotation.
(B) `` S _{ N } 1 `` reaction yields `` 1: 1 `` mixture of both enantiomers.
(C)The product obtained by `` S _{ N}2 `` reaction of haloalkane having chirality at the reactive site shows inversion of configuration.
(D)Enantiomers are superimposable mirror images on each other.digAnsr: DAns : Enantiomers are non-superimposable mirror images of each other.
- Qstn #32The IUPAC name of an element with atomic number 119 is
(A)ununoctium
(B)ununennium
(C)unnilennium
(D)unununniumdigAnsr: BAns : IUPAC nomenclature
`` 119 \rightarrow \text { Ununennium } \rightarrow \text { Uue } ``
- Qstn #33Which statement regarding polymers is not correct?
(A)Thermosetting polymers are reusable.
(B)Elastomers have polymer chains held together by weak intermolecular forces.
(C)Fibers possess high tensile strength.
(D)Thermoplastic polymers are capable of repeatedly softening and hardening on heating and cooling respectively.digAnsr: AAns : Thermosetting polymers are NOT reusable.
- Qstn #34Given below are two statements : Statement I: The boiling points of the following hydrides of group `` 16 `` elements increases in the order - `` H _{2} O < H _{2} S < H _{2} Se < H _{2} Te \text {. } `` Statement II : The boiling points of these hydrides increase with increase in molar mass. In the light of the above statements, choose the most appropriate answer from the options given below :
(A)Statement I is incorrect but Statement II is correct
(B)Both Statement I and Statement II are correct
(C)Both Statement I and Statement II are incorrect
(D)Statement I is correct but Statement II is incorrectdigAnsr: AAns : Hydrides of group `` 16^{\text {th }} ``
`` \underset{\text{H-bond}}{H _{2} O} \,\,\, \underbrace{ H _{2} S \quad H _{2} Se \quad H _{2} Te }_{ H \text {-bond }} ``
B.P. `` \rightarrow H _{2} S < H _{2} Se < H _{2} Te < H _{2} O ``
- Qstn #35Amongst the following which one will have maximum 'lone pair - lone pair' electron repulsions?
(A) `` XeF _{2} ``
(B) `` ClF _{3} ``
(C) `` IF _{5} ``
(D) `` SF _{4} ``digAnsr: AAns :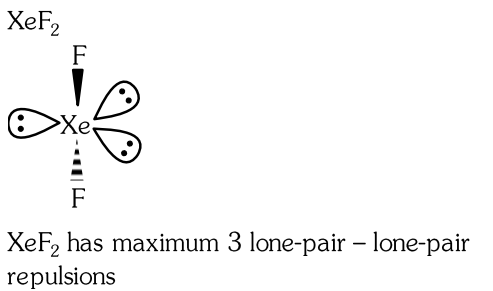
- Qstn #36For a first order reaction `` A \rightarrow `` Products, initial concentration of `` A `` is `` 0.1 \,M `` , which becomes `` 0.001 \,M `` after `` 5 `` minutes. Rate constant for the reaction in `` \min ^{-1} `` is
(A) `` 0.2303 ``
(B) `` 1.3818 ``
(C) `` 0.9212 ``
(D) `` 0.4606 ``digAnsr: CAns : A `` \rightarrow `` Products
Initial conc. `` A _{ o }=0.1 \,M ``
Conc. After `` 5 \min A_{t}=0.001\, M ``
`` t =5 \min `` .
For first order reaction
`` K =\frac{2.303}{ t } \log \left(\frac{ A _{ o }}{ A _{ t }}\right) ``
`` =\frac{2.303}{5} \log \left(\frac{0.1}{0.001}\right) ``
`` K =0.9212 \min ^{-1} ``
- Qstn #37Which one of the following is not formed when acetone reacts with `` 2 `` -pentanone in the presence of dilute `` NaOH `` followed by heating ?
(A)
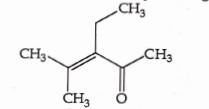
(B)
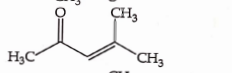
(C)
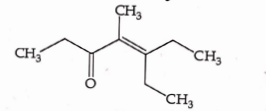
(D)
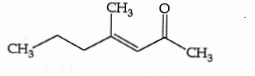 digAnsr: CAns :
digAnsr: CAns :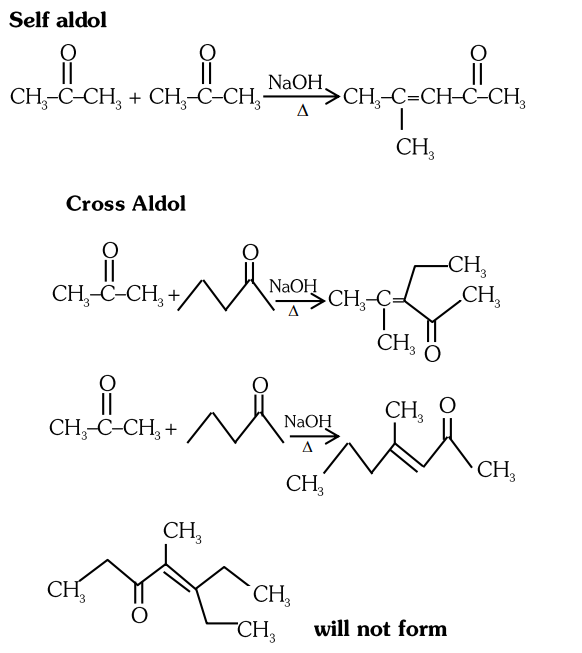
- Qstn #38In the neutral or faintly alkaline medium, `` KMnO _{4} `` oxidises iodide into iodate. The change in oxidation state of manganese in this reaction is from
(A) `` +6 `` to `` +5 ``
(B) `` +7 `` to `` +4 ``
(C) `` +6 `` to `` +4 ``
(D) `` +7 `` to `` +3 ``digAnsr: BAns :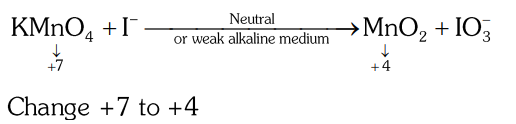
- Qstn #39Compound `` X `` on reaction with `` O _{3} `` followed by `` Zn `` / `` H _{2} O `` gives formaldehyde and `` 2 `` -methyl propanal as products. The compound `` X `` is :
(A)Pent-2-ene
(B)3-Methylbut-1-ene
(C)2-Methylbut-1-ene
(D)2-Methylbut-2-enedigAnsr: BAns :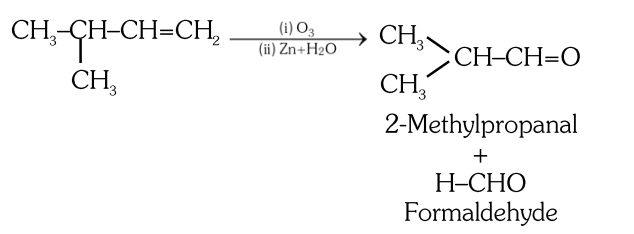
- Qstn #40`` 3 O _{2}( g ) \rightleftharpoons 2 O _{3}( g ) `` for the above reaction at `` 298\, K , K _{ c } `` is found to be `` 3.0 \times 10^{-59} `` . If the concentration of `` O _{2} `` at equilibrium is `` 0.040 \,M `` then concentration of `` O _{3} `` in `` M `` is
(A) `` 1.2 \times 10^{21} ``
(B) `` 4.38 \times 10^{-32} ``
(C) `` 1.9 \times 10^{-63} ``
(D) `` 2.4 \times 10^{31} ``digAnsr: BAns : `` 3 O _{2}( g ) \rightleftharpoons 2 O _{3}( g ) ``
`` K _{ c }=\frac{\left[ O _{3}\right]^{2}}{\left[ O _{2}\right]^{3}} ``
`` 3 \times 10^{-59}=\frac{\left[ O _{3}\right]^{2}}{\left(4 \times 10^{-2}\right)^{3}} ``
`` {\left[ O _{3}\right]^{2}=3 \times 10^{-59} \times 64 \times 10^{-6} } ``
`` =19.2 \times 10^{-64} ``
`` =4.38 \times 10^{-32} M ``
- Qstn #41If radius of second Bolur orbit of the `` He ^{+} `` ion is `` 105.8 `` `` pm `` , what is the radius of third Bohr orbit of `` Li ^{2+} `` ion?
(A) `` 158.7\,Å ``
(B) `` 158.7 \,pm ``
(C) `` 15.87\, pm ``
(D) `` 1.587 \,pm ``digAnsr: AAns :
`` \frac{\left( r _{3}\right)_{ L ^{+2}}}{\left( r _{2}\right)_{ He ^{+}}}=\frac{ n _{1}^{2}}{ n _{2}^{2}} \times \frac{ Z _{2}}{ Z _{1}} ``
`` \frac{\left( r _{3}\right)_{ L ^{+2}}}{105.8\, pm }=\frac{3 \times 3}{2 \times 2} \times \frac{2}{3} ``
`` \left( r _{3}\right)_{ Li ^{+2}}=158.7\, pm ``
- Qstn #42The pollution due to oxides of sulphur gets enhanced due to the presence of : (a) particulate matter (b) ozone (c) hydrocarbons (d) hydrogen peroxide Choose the most appropriate answer from the options given below:
(A)
(a),
(c),
(d) only
(B)
(a),
(d) only
(C)
(a),
(b),
(d) only
(D)
(b),
(c),
(d) onlydigAnsr: CAns : The presence of particulate matter in polluted air catalyses the oxidation of sulphurdioxide to sulphur trioxide.
`` 2 SO _{2}( g )+ O _{2}( g ) \rightarrow 2 SO _{3}( g ) ``
The reaction can also be promoted by ozone and hydrogen peroxide.
`` SO _{2}( g )+ O _{3}( g ) \rightarrow SO _{3}( g )+ O _{2}( g ) ``
`` SO _{2}( g )+ H _{2} O _{2}( l ) \rightarrow H _{2} SO _{4}( aq ) ``
- Qstn #43A `` 10.0 \,L `` flask contains `` 64\, g `` of oxygen at `` 27^{\circ} C `` . (Assume `` O _{2} `` gas is behaving ideally). The pressure inside the flask in bar is (Given `` R =0.0831 \,L\, bar\, K ^{-1} mol ^{-1} `` )
(A) `` 4.9 ``
(B) `` 2.5 ``
(C) `` 498.6 ``
(D) `` 49.8 ``digAnsr: AAns : `` V =10 L ``
`` W _{ O _{2}}=64 \,g ``
`` T =2{ }^{\circ} C ``
`` n _{ O _{2}}=2 ``
`` R =0.083 . L `` bar `` K ^{-1} \,mol ^{-1} ``
Ideal gas equation `` PV = nRT ``
`` P =\frac{2 \times 0.0831 \times 300}{10} ``
`` P =4.9 `` bar
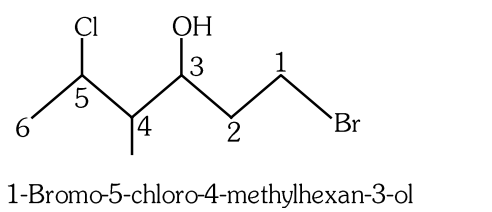
- Qstn #44The correct IUPAC name of the following compound is:
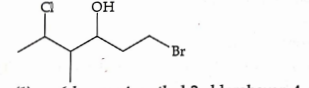
(A)6-bromo-4-methyl-2-chlorohexan-4-ol
(B)1-bromo-5-chloro-4-methylhexan-3-ol
(C)6-bromo-2-chloro-4-methylhexan-4-ol
(D)1-bromo-4-methyl-5-chlorohexan-3-oldigAnsr: BAns :