NEET-XII-Chemistry
Previous Year Paper year:2018
- Qstn #75Which oxide of nitrogen is not a common pollutant
introduced into the atmosphere both due to natural
and human activity ?
(1) ``\ce N2O5``
(2) ``NO_2``
(3) ``N_2O``
(4) NOdigAnsr: 1Ans : (1)
Sol. Nitrous oxide (N2O) occurs naturally in environment.
In automobile engine, when fossil is burnt dinitrogen
& dioxygen combine to yield NO & NO2.
- Qstn #76For the redox reaction
``\ce MnO4^-
+ C2O4^{2-}+ H^+
\longrightarrow Mn^{2+} +CO2 + H2O``
the correct coefficients of the reactants for the
balanced equation are
``MnO_4^{-}`` ``C_2O_4^{2-}`` `` H^+``
(1) 16 5 2
(2) 2 5 16
(3) 2 16 5
(4) 5 16 2digAnsr: 2Ans : (2)
Sol.
7
2
4MnO Mn ; 5e gain
+
+ (1)
+ +
3 4
2
2 4 2C O CO ; 2e loss (2)
multiplying (1) by 2 and (2) by 5 to balance e-
++ +2 24 2 4 22MnO 5C O 2Mn 10CO
on balancing charge;
+ ++ + + +2 24 2 4 2 22MnO 5C O 16H 2Mn 10CO 8H O
15
CODE - PP
- Qstn #77Which one of the following conditions will favour
maximum formation of the product in the reaction,
``A_2(g) + B_2(g) \leftrightarrows X_2(g) \triangle_r``H = -X kJ ?
(1) Low temperature and high pressure
(2) Low temperature and low pressure
(3) High temperature and high pressure
(4) High temperature and low pressuredigAnsr: 1Ans : (1)
Sol. For reaction ▵H = - ve and ▵ng = - ve
∴ High P, Low T, favour product formation.
- Qstn #78The correction factor 'a' to the ideal gas equation
corresponds to
(1) density of the gas molecules
(2) volume of the gas molecules
(3) electric field present between the gas molecules
(4) forces of attraction between the gas moleculesdigAnsr: 4Ans : (4)
Sol. Vanderwaal constant (a) forces of attraction.
- Qstn #79When initial concentration of the reactant is doubled,
the half-life period of a zero order reaction
(1) is halved (2) is doubled
(3) is tripled (4) remains unchangeddigAnsr: 2Ans : (2)
Sol.
= 01 / 2 zero
A
t
2K
∴ If [A]0 = doubled, t1/2 = doubled
- Qstn #80The bond dissociation energies of ``X_2, Y_2 and XY``
are in the ratio of 1 : 0.5 : 1.
``\triangle``H for the formation
of XY is -200 kJ ``mol^{-1}``. The bond dissociation
energy of ``X_2`` will be
(1) 200 kJ ``mol^{-1}``
(2) 100 kJ ``mol^{-1}``
(3) 800 kJ ``mol^{-1}``
(4) 400 kJ ``mol^{-1}``digAnsr: 3Ans : (3)
Sol. let B.E. of x2 , y2 & xy are x kJ mol-1,
0.5x kJ mol-1 and x kJ mol-1 respectively
+ ▵ = 12 2
1 1
x y xy; H 200 kJmol
2 2
▵H = - 200 = (B.E)Reactant - (B.E)Product
= +
1 1
x 0.5x - 1 x
2 2
B.E of X2 = x = 800 kJ mol
-1
- Qstn #81Identify the major products P, Q and R in the
following sequence of reaction :

(1)
(2)
(3)
(4)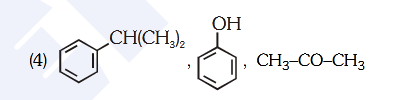 digAnsr: 4Ans : (4)
digAnsr: 4Ans : (4)
Sol.
Mech: CH -CH -CH -Cl3 2 2
AlCl3 CH -CH -CH +AlCl3 2 2 4
CH
CH3 CH3
(P)
ESR
CH -CH-CH3 3
H
shift
-
CH
CH3 CH3
Cumene
▵
C-O-O-HCH3
CH3
Cumene
Hydroperoxide
H O3
+
▵
O2
(P)
OH
Phenol
+ CH -C-CH3 3
O
Acetone
(Q)
(R)
- Qstn #82Which of the following compounds can form a
zwitterion ?
(1) Aniline
(2) Acetanilide
(3) Benzoic acid
(4) GlycinedigAnsr: 4Ans : (4)
Sol. The molecule which forms zwitter ion is glycine.
HOOC-CH2 - NH2 O
-
OC-CH2 -
N H3
Zwitter ion
- Qstn #83The type of isomerism shown by the complex
[``CoCl_2(en)_2``] is
(1) Geometrical isomerism
(2) Coordination isomerism
(3) Ionization isomerism
(4) Linkage isomerismdigAnsr: 1Ans : (1)
Sol. Co
Cl
en en
Cl
Co en
Cl
Cl
en
Trans cis
- Qstn #84Which one of the following ions exhibits d-d
transition and paramagnetism as well ?
(1) ``CrO^{2-}_4``
(2) ``Cr_2O^{2-}_7``
(3) ``MnO_4^{-}``
(4) ``MnO_4^{2-}``digAnsr: 4Ans : (4)
Sol. CrO4-2 Cr+6 diamagnetic
Cr2O7
-2 Cr+6 diamagnetic
MnO4
- Mn+7 diamagnetic
MnO4
-2 Mn+6 paramagnetic
unpaired electron is present so d-d transition is
possible.
Before
transition
After
transition
- Qstn #85The geometry and magnetic behaviour of the
complex [``Ni(CO)_4``] are
(1) square planar geometry and diamagnetic
(2) tetrahedral geometry and diamagnetic
(3) square planar geometry and paramagnetic
(4) tetrahedral geometry and paramagneticdigAnsr: 2Ans : (2)
Sol. tetrahedral geometry and diamagnetic
Ni 3d8 4s2
CO is SFL so unpaired electrons will get paired.
CO CO CO CO
sp3 hybridisation
Tetrahedral, diamagnetic
- Qstn #86Iron carbonyl, ``Fe(CO)_5`` is
(1) tetranuclear
(2) mononuclear
(3) trinuclear
(4) dinucleardigAnsr: 2Ans : (2)
Sol. Fe(CO)5
EAN = Z-O.N. + 2(C.N.)
= 26 - 0 + 2(5)
= 26 + 10
= 36
only one central metal atom/ion is present and it
follows EAN rule, so it is mononuclear
- Qstn #87Match the metal ions given in Column I with the spin
magnetic moments of the ions given in Column II
and assign the correct code :
Column I Column II
a.`` Co^{3+}`` i. ``\sqrt8`` B.M.
b. ``Cr^{3+}`` ii. ``\sqrt35`` B.M.
c. ``Fe^{3+}`` iii. ``\sqrt3`` B.M.
d. ``Ni^{2+}`` iv. ``\sqrt24`` B.M.
v. ``\sqrt24`` B.M.
a b c d
(1) iv v ii i
(2) i ii iii iv
(3) iv i ii iii
(4) iii v i iidigAnsr: 1Ans : (1)
17
CODE - PP
Sol. Magnetic moment () = +n n 2 B.M.
(a) Co3+ 1s2 2s2 2p6 3s2 3p6 4s0 3d6
n = 4
= + =4 4 2 24 B. M
(b) Cr+3 1s2 2s2 2p6 3s2 3p6 4s0 3d3
n = 3
= + =3 3 2 15 B.M.
(c) Fe3+ 1s2 2s2 2p6 3s2 3p6 4s0 3d5
n = 5
= + =5 5 2 35 B. M.
(d) Ni+2 1s2 2s2 2p6 3s2 3p6 4s0 3d8
n = 2
= + =2 2 2 8 B. M.
- Qstn #88Which of the following is correct with respect to
-I effect of the substituents ? (R = alkyl)
(1)``\ce - NH2 < - OR < - F``
(2) ``\ce -NR2 < - OR < - F``
(3) ``\ce - NH2 > - OR > - F``
(4) ``\ce - NR2 > - OR > - F``digAnsr: 1Ans : (1/2)
Sol. (Based on EN)
∴ -NH2 < -OR < -F -I effect
Also -NR2 < -OR < -F -I effect
- Qstn #89Which of the following carbocations is expected to
be most stable ?
(1)
(2)
(3)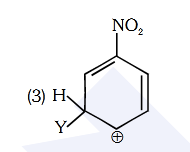
(4)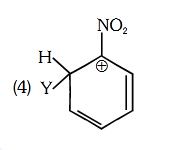 digAnsr: 3Ans : (3)
digAnsr: 3Ans : (3)
Sol. -NO2 group is meta-directing group
Y H Y H
NO2NO2
(Less stable due to more e- withdrawing effect of
-NO2)
H
Y
NO2NO2
H
Y
NO2
H
Y
(More stable due to less e- withdrawing effect of
-NO2)